On 24 April 2024, the Food and Drug Administration of Thailand (FDA) issued a consultation document proposing to revise the standards for olive oil and pomace oil, with a feedback period ending on 14 June 2024, for comments. The main contents are as follows:
(1) Revision of olive oil and pomace oil definitions; e.g. olive oil oleic acid content ≤ 0.8g/100g; virgin olive oil oleic acid content ≤ 2g/100g, etc;
(2) Quality standards. Physicochemical properties: saponification value: 184-196mgKOH/g; iodine value: 75-94mg/g; unsaponifiables: 15g/kg;
(3) Revision of the standards for the content of various fatty acids in olive oil, some of which are shown in the table below.
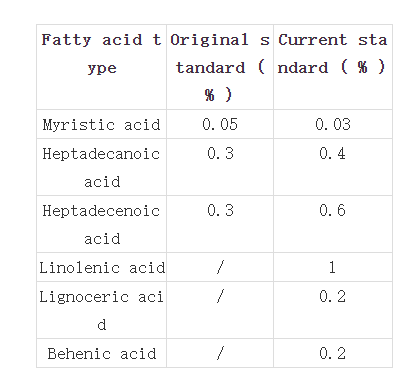
For more information see: https://food.fda.moph.go.th/press-release/law-technical/, https://drive.google.com/file/d/1jx7Xj8sQhtrELuvCJS_EoXhbvT__KQuo/ view?pli=1